Association of blood glucose control and pancreatic reserve with Diabetic Retinopathy (DR) in the Veterans Affairs Diabetes Trial (VADT), Azad N,Agrawal L,Emanuele NV,et al. Diabetologia.2014;57(6):1124-31
Key objective:
Testing the hypothesis that, Intensive Glycemic Control (INT) and higher plasma C-peptide levels in patients with poorly controlled diabetes would be associated with better eye outcomes.
Results:
Risk of progression (but not incidence) of DR increased by 30% for each 1% increase in baseline HbA1c (p=0.0004). Neither assignment to INT nor age was independently associated with DR in the entire cohort. However, INT showed a biphasic interaction with age. The incidence of DR was decreased in INT participants =55 years of age but increased in those =70 years old p=0.0043. The incidence of DR was reduced by 67.2% with each 1 pmol/ml increment in baseline C-peptide (p=0.0037). Baseline C peptide was also an independent inverse risk factor for the progression of DR, with a reduction of 47% with each 1 pmol/ml increase in C-peptide (p=0.0244).
Clinical take home message:
Poor glucose control at baseline was associated with an increased risk of progression of DR. INT was associated with a decreased incidence of DR in younger patients but with an increased risk of DR in older patients. Higher C-peptide at baseline was associated with reduced incidence and progression of DR.
CAN is independently associated with CKD, albuminuria and eGFR in patients with type 2 diabetes. In addition, CAN is an independent predictor of the decline in eGFR over the follow-up period. CAN could be used to identify patients with type 2 diabetes who are at increased risk of rapid decline in eGFR, so that preventative therapies might be intensified.
SGLT-2 Inhibitors: A New Mechanism for Glycemic Control
Sodium-glucose Co-transporter 2 (SGLT-2) mediates glucose reabsorption in the kidney by the active transport of glucose (against a concentration gradient) across the luminal membrane by coupling it with the downhill transport of Na+. Glucose excretion can be induced by blocking the activity of the renal SGLT-2. By lowering the renal threshold for glucose excretion, SGLT-2 inhibitors suppress renal glucose reabsorption and thereby increase Urinary Glucose Excretion (UGE). Phase 2 and 3 clinical trials of SGLT-2 inhibitors used as add-on therapy with other drugs demonstrated improved glycemic control with low rates of hypoglycemia. Given the Insulin-independent mechanism of action of SGLT-2 inhibitors, hypoglycemia would not be expected and indeed, very low rates of hypoglycemia have been observed in clinical trials. Events suggestive of UTI (Urinary Tract Infection) are reported more often in female patients receiving SGLT-2 inhibitors than in male patient
Clinical take home message:
SGLT-2 inhibitors have a novel mechanism of action, blocking glucose reabsorption that is independent of Insulin secretion and action with an advantage of reduced hyperglycemia without hypoglycemia, along with weight loss and blood pressure reduction, these findings were demonstrated from multiple phase III studies of > 5,000 subjects. Taken together, the clinical evidence to date suggests that SGLT-2 inhibitors hold promise as an important addition to the toolbox of treatment options for type 2 diabetes.
The U.S FDA has approved the inhaled human insulin product Afrezza to improve glycemic control in adults with type 1 or type 2 diabetes.The FDA said the device offers a new treatment option for patients with type 1 diabetes.
The approval broadens the options available for delivering mealtime insulin for diabetes patients. The drug will carry a boxed warning that acute bronchospasm has been seen in patients with asthma and chronic obstructive pulmonary disease and that it should not be used in patients with those conditions. Afrezza is a rapid-acting inhaled insulin to be administered prior to meals or within 20 minutes of starting a meal. It is not a substitute for long-acting insulin and must be used in combination with long-acting insulin in patients with type 1 diabetes. It is not recommended for the treatment of diabetic ketoacidosis or in patients who smoke or who have chronic lung disease. Efficacy and safety data came from studies involving a total of 3,017 patients, including 1,026 with type 1 and 1,991 with type 2 diabetes. At 24 weeks, Afrezza reduced HbA1clevels by the prespecified end point of 0.4% points in both groups. Hemoglobin A1c reduction was inferior to that of insulin aspart among type 1 patients but significantly superior to placebo among type 2 patients who were also taking oral glucose-lowering medications. The most common adverse reactions associated with Afrezza in clinical trials were hypoglycemia, cough, and throat pain or irritation. Afrezza will come in a powder form with a small inhaler which is pocket sized and easy to train and use. Different doses come in multiple cartridges which each contain a single dose that is disposable after use. Afrezza may also not be available for a while as MannKind is seeking a partner with a pharmaceutical company for distribution.
Evaluation of maternal and neonatal safety of Metformin in Gestational Diabetes Mellitus (GDM)
Results:
Among the 186 clinical records reviewed of GDM women, 17.2% underwent Metformin during pregnancy. No statistical differences between diet and Metformin groups were found with regard to the rates of abortion, prematurity, preeclampsia, macrosomy, small-for-gestational-age or Large-for Gestational-Age (LGA) newborns, cesarean deliveries, neonatal intensive care unit admissions and birth malformations or neonatal injuries. Similarly, there were no differences between Metformin and Insulin groups with regard to the referred outcomes. No abortions or perinatal deaths were recorded in the Metformin group. Ten out of 32 patients under Metformin required additional Insulin.
Clinical take home message:
Metformin is a safe alternative or additional treatment to Insulin for GDM women. Metformin was not associated with a higher risk of maternal or neonatal complications when compared to Insulin or to diet groups.


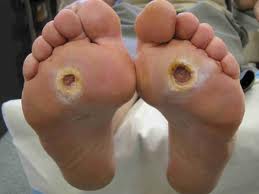 Diabetic Foot
Diabetic Foot